Basic Chemicals

SODIUM BICARBONATE
- 1. CAS No.: 144-55-8
- 2. Appearance: White crystal powder
- 3.Purity: 99%MIN
The product, Sodium Bicarbonate, is used as food fermentation. pharmacy, feather, ore dressing, metallurgy, fire extinguishing agent, espacially in reducing stomach acid, decreasing acid in the blood and urined, ecreasing potassium levels in the body.
The Specification
| Item | specification |
| Appearance | White crystal powder |
| Total alkali content (as NaHCO3) | 99.0%-100.5% |
| loss on drying | 0.2% max |
| Arsenic | 1ppm max |
| Heavy metals as Pb | 5ppm max |
| pH value | 8.6 max |
| Chloride content | 0.10% max |
| Iron Content | 0.001% max |
| Ammonium salt | pass |
| Clearness | pass |
| Molecular formula | NaHCO3 |
| Alias | Baking soda, bicarbonate and sodium hydrogen carbonate |
| Variety | Edible baking soda, feed grade baking soda and fine baking soda |
| Quality standard | Industrial grade GB/T 1606-2008 and food grade GB 1887-2007 |
| Use | It is a bulking agent widely applied to the food industry. It is used to produce biscuits, cookies, steamed buns and bread. It is a generator of carbon dioxide in soda water. It can be combined with alum into alkaline baking powder, soda into civilian crystal soda, or used as a butter preservative. It can produce acid-alkali and foam fire extinguishers, rubber and sponge, and used with alum and H pore-forming agent to form pores in the rubber industry. In metallurgical industry, it is used as a fluxing agent for steel ingot casting, a sand-mold molding additive of steel casting in mechanical industry, color fixing agent, acid–alkali buffer and fabric finishing agent in the printing and dyeing industry, and raw material of antacids in the pharmaceutical industry. |
| Properties | It is white crystalline powder, odorless and salty, with density of 2. 159g/cm3. It is soluble in water but ethanol. Its water solution is slightly alkaline. It is stable in dry air and can be slowly decomposed in humid air. It will be hydrolyzed completely above 270℃, release carbon dioxide and become sodium carbonate. |
| Production method | It is obtained after concentrated sodium carbonate solution or crystalline sodium carbonate absorbs carbon dioxide. |
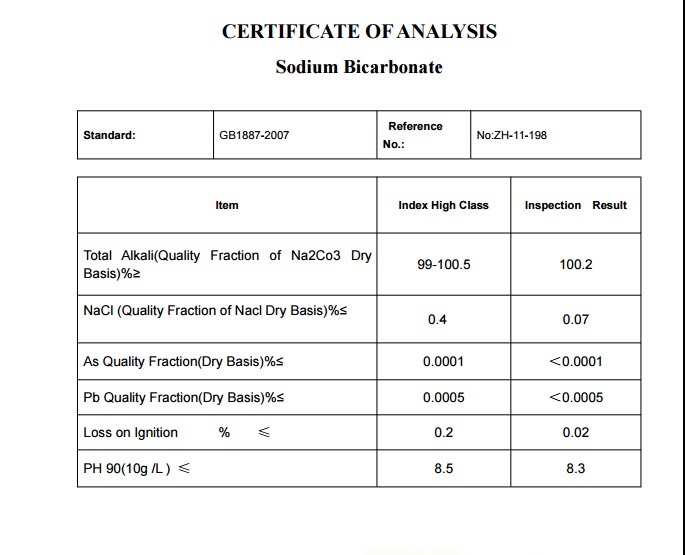
CERTIFICATE OF ANALYSIS
GOODS PHOTO

PACKING: BAG PHOTO